The safety of patients and medical staff in medical use rooms is the subject of standard DIN VDE 0100-710 "Errichten von Niederspannungsanlagen, Anforderungen an Betriebsstätten, Räumen und Anlagen besonderer Art, Teil 710: medizinisch genutzte Räume" of October 2012. In parallel, the IEC is working on the corresponding standard IEC 60364-7-710 “Electrical installations – Part. 7-7-10 Requirements for special installations or locations – Medical locations” whose current state of deliberation has not yet attained the safety level for the generally high-level medical equipment in Germany. At European level (CENELEC), there is harmonisation document HD 60364-7-710:2012 vor, which DIN VDE 0100-710 refers to.
Requirements on medical rooms
Medical use rooms include:
hospitals and clinics, container constructions included;
sanatoriums and wellness clinics;
care homes and homes for the elderly;
medical centres, polyclinics and outpatient clinics;
medical practices and dentists’ surgeries; and
other outpatient facilities e.g. operated by industrial and sports physicians as well as other medical doctors.
Medical use areas are divided up into groups depending on the exposure of humans and animals to electrical equipment or components thereof (examples see table 6.6):
Group 0: No physical exposure to components of medical devices
Group 1: External or invasive exposure to components of medical devices
Group 2: Direct exposure of the heart (intracardiac exposure) to components of electrical equipment, e.g. in the operating theatre or during vital treatments which can cause danger to life in case of power supply malfunctions.
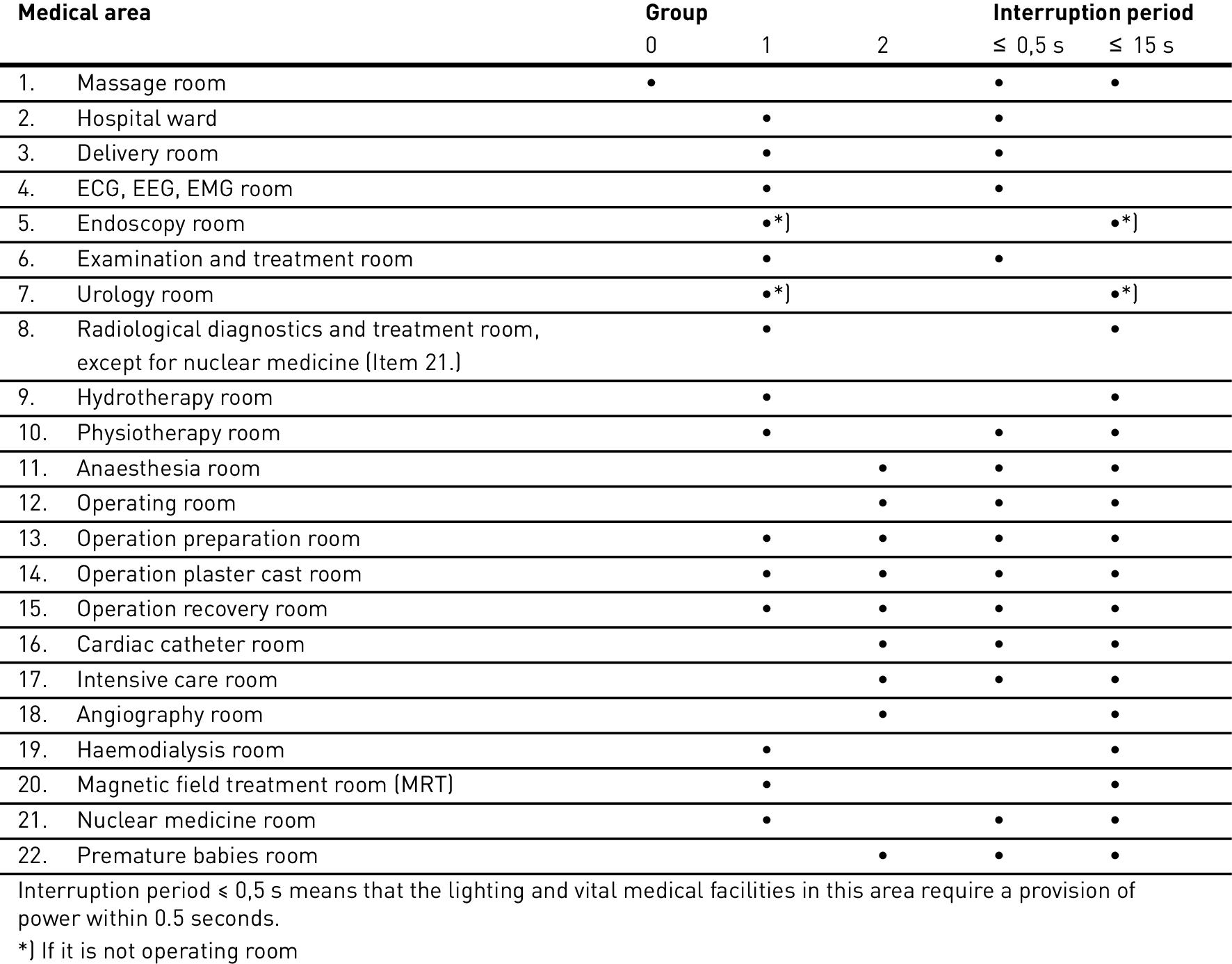
Table 3.164: Categorisation of medical areas into groups 0, 1 and 2 as well as permissible interruption period of general power supply before the emergency power supply according to DIN VDE 0100-710 activates.